Working toward the next evolution in diabetes technology
In recent years, continuous glucose monitoring devices (CGMs) have revolutionized diabetes management, offering numerous advantages over traditional point-in-time glucose monitoring methods. However, several challenges remain—including consistency in accuracy, the need for calibration, and concerns related to comfort and wearability.
Glucotrack is investigating a novel approach that draws from both continuous glucose monitoring and blood glucose monitoring methodologies. Our investigational system is being developed with the goal of combining the direct measurement of blood glucose with the data frequency of continuous monitoring, while seeking to overcome some of the limitations associated with wearable technologies.
Our CBGM is being developed with the goal of reducing device-related burden and supporting a more seamless daily experience for people living with diabetes.
Exploring new possibilities
Glucotrack is developing a fully implantable investigational continuous blood glucose monitoring (CBGM) system—a small device designed to be placed under the skin and evaluated for its ability to continuously measure blood glucose for up to 3 years.* The system is being evaluated for its potential to reduce calibration needs and address challenges related to comfort and wearability often associated with current CGM technologies.
With a focus on people-centered design, our goal is to create a solution that may help make glucose monitoring less burdensome for individuals living with diabetes.
Exploring new possibilities
Glucotrack is developing a fully implantable investigational continuous blood glucose monitoring (CBGM) system—a small device designed to be placed under the skin and evaluated for its ability to continuously measure blood glucose for up to 3 years.* The system is being evaluated for its potential to reduce calibration needs and address challenges related to comfort and wearability often associated with current CGM technologies.
With a focus on people-centered design, our goal is to create a solution that may help make glucose monitoring less burdensome for individuals living with diabetes.

Sustainable by design
Traditional diabetes devices, especially CGMs, generate a steady stream of plastic, electronics, batteries, sharps, and packaging waste. Studies estimate that current CGM use contributes over 20,000 tons of annual global waste, including 340 tons of plastic.1 In the US, people with diabetes using CGMs and injection therapies generate up to 3 pounds of device waste per month.2
Glucotrack’s implantable CBGM is fundamentally different by design. With no disposable sensors, no adhesives, and no external wearables to replace every 7-15 days, the system reduces material waste at the source. Its small size, long-term use, and absence of daily disposables help reduce the environmental impact of diabetes management—simply by how it works.
CBGM investigational design highlights
Fully implantable system.
All components are designed to be placed beneath the skin, with the intent of addressing the limitations associated with wearable sensors.
Long-term use.
The system is being studied for continuous blood glucose measurement for up to 3 years.*
Blood-based sensing.
The CBGM is intended to measure glucose directly from the blood in real time—an approach that may help address the time lag associated with interstitial fluid readings.
Real-time monitoring.
The system is being evaluated for its accuracy over time.
Low-burden design.
Our engineers designed this device with minimal user calibration in mind, aiming to improve user experience.
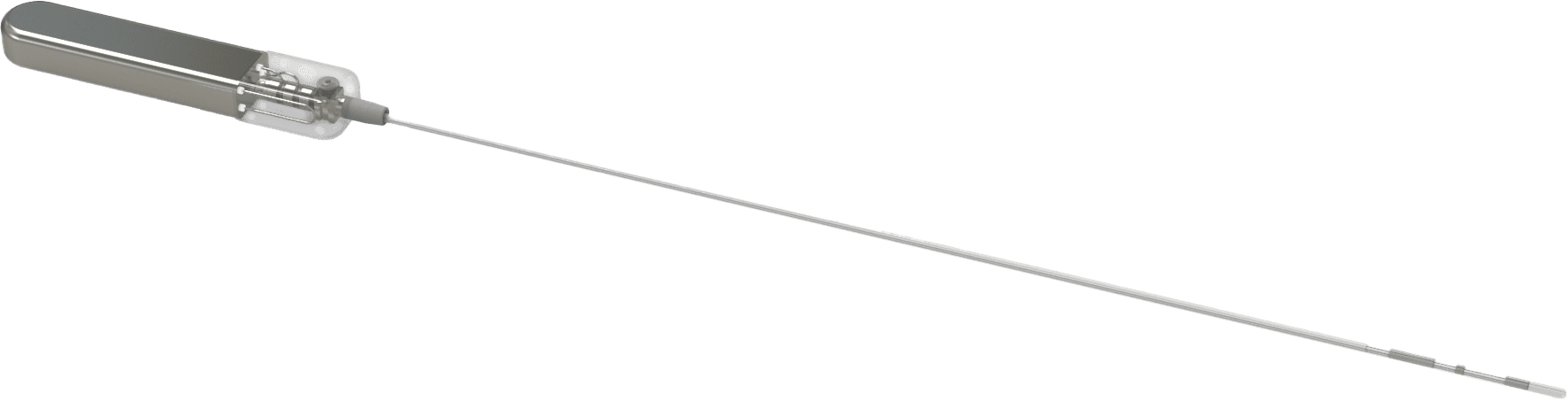
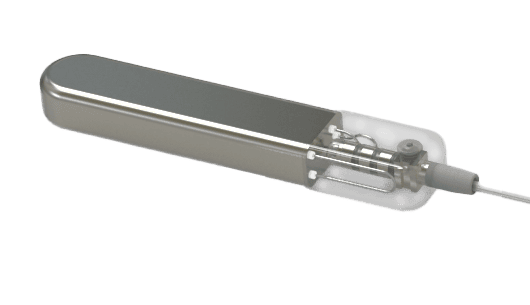
Latest version of our CBGM prototype. This fully implantable investigational device is being evaluated for its ability to provide continuous blood glucose monitoring for up to 3 years.*
*Pending FDA submission and review. Glucotrack intends to initially seek regulatory approval for 12-month sensor duration. While the device has been designed for use up to 36 months, future submissions may request approval for extended use based on additional clinical data.
Exploring broader application
With the global rise in diabetes and related chronic conditions, Glucotrack is investigating whether its CBGM system could be applicable in other therapeutic areas. One area of early stage research includes Painful Diabetic Neuropathy (PDN), a progressive neurological complication affecting roughly 1 in 5 Americans living with diabetes. PDN can cause symptoms such as pain and numbness in the extremities, which may impact daily functioning and comfort. As part of our ongoing research, we are evaluating the feasibility of continuous glucose sensing in the epidural space to better understand its potential relevance in PDN management.
Development milestones
Leveraging techniques from the cardiovascular field, the investigational CBGM system includes a transmitter designed for subcutaneous placement and a sensor designed for intravascular placement. In clinical studies, placement is performed by trained interventionists with expertise in cardiac procedures, using established interventional techniques.
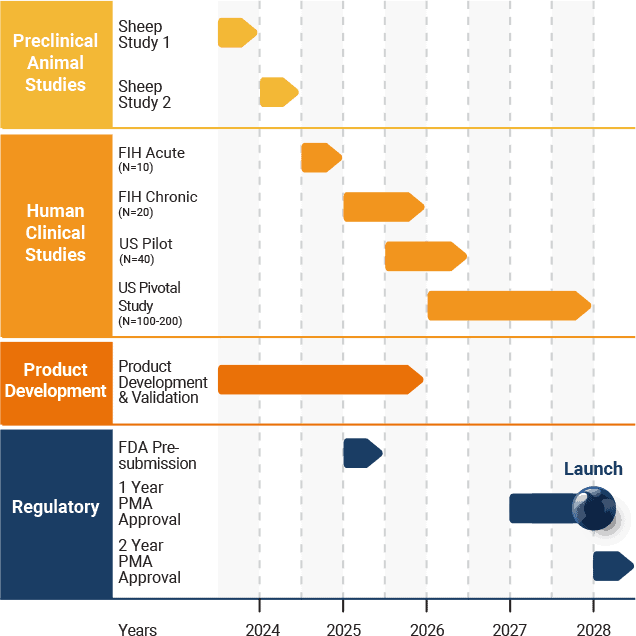
View the latest Investor Presentation for updated development milestones.
References: 1. Petry SF, Zaharieva DP, Turksoy K, McGaugh SM, Renard E, Castle JR. Quantification of different types of waste and batteries associated with the widespread usage of continuous glucose monitoring systems. Journal of Diabetes Science and Technology. Published online December 21, 2023. doi:10.1177/19322968241305161 2. Tian Y, Kim JH, Paterson M, Zhao J, Foster NC, Miller KM, Shah VN. Quantifying environmental waste from diabetes devices in the United States: a T1D exchange observational study. Diabetes Care. 2025;48(7):1198-1203. doi:10.2337/dc24-2522
Glucotrack’s implantable CBGM is an investigational device and has not been approved by the FDA. Safety and effectiveness have not been established.